 Salix Pharmaceuticals may soon bring a new treatment for mild-to-moderate distal ulcerative colitis to the market with its tentative Food and Drug Administration (FDA) approval for UCERIS (budesonide) rectal foam. The product, which is a rectally-administered corticosteroid foam, addresses limitations of other currently approved therapies.
Salix Pharmaceuticals may soon bring a new treatment for mild-to-moderate distal ulcerative colitis to the market with its tentative Food and Drug Administration (FDA) approval for UCERIS (budesonide) rectal foam. The product, which is a rectally-administered corticosteroid foam, addresses limitations of other currently approved therapies.
“The unique delivery system of UCERIS rectal foam can help overcome current treatment limitations by reaching the affected area of the distal colon and keeping the medication there long enough to be effective,” said Bill Forbes, Executive Vice President of Medical and Research Development and Chief Development Officer of Salix, in a news release from the company. “People suffering from distal ulcerative colitis now have another option to consider when facing this disease.”
In fact, rectal therapy is the recommended means for inducing remission in patients with mild-to-moderate ulcerative proctitis and patients with mild-to-moderate ulcerative colitis. Enemas and suppositories used today can be difficult to administer and provide a small spread of distribution to afflicted areas of the colon. Although UCERIS was not tested head-to-head against these other treatments, it was evaluated through a number of preclinical studies and clinical trials.
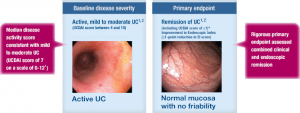
 The most recent study leading to tentative FDA approval was a phase 3 clinical trial of patients receiving 2 mg UCERIS rectal foam or placebo. At the end of six weeks of treatment, 41.2% of UCERIS-treated patients achieved remission of distal ulcerative colitis, and only 24.0% of placebo-treated patients achieved remission. The difference was significant and was further supported by differences in Modified Mayo Disease Activity Index endoscopy score and less rectal bleeding.
The most recent study leading to tentative FDA approval was a phase 3 clinical trial of patients receiving 2 mg UCERIS rectal foam or placebo. At the end of six weeks of treatment, 41.2% of UCERIS-treated patients achieved remission of distal ulcerative colitis, and only 24.0% of placebo-treated patients achieved remission. The difference was significant and was further supported by differences in Modified Mayo Disease Activity Index endoscopy score and less rectal bleeding.
“These trials definitively demonstrated that UCERIS rectal foam is an efficacious and well-tolerated rectal therapy for the induction of remission in patients with mild-to-moderate distal ulcerative colitis,” said William Sandborn, MD, principal investigator on the trial and Director of the UCSD IBD Center. “Treatment with UCERIS rectal foam also led to a rapid response for rectal bleeding that was sustained through the sixth week of the trial.”
Tentative approval indicates that UCERIS meets all manufacturing quality and clinical and safety efficacy standards required by the FDA. Once patent issues are resolved, the FDA will grant final approval. Salix may launch UCERIS rectal foam as soon as the first quarter of 2015, given patent issues are resolved early in the fourth quarter of 2014.
A new product on the market may provide relief to a wide range of patients. Approximately 46% of ulcerative colitis patients have distal ulcerative colitis. “Distal ulcerative colitis can be challenging for patients to live with and gastroenterologists to treat,” commented Mr. Forbes. “Our goal is to put the disease into remission so that patients can get on with their lives.”

