 Celltrion recently announced it has received approval from the U.S. Food and Drug Administration (FDA) for the use of its Inflectra (biosimilar infliximab) product for all indications of the reference product, Janssen Biotech Inc.’s Remicade (infliximab), originally licensed in 1998. Inflectra is sold in Europe as Remsima.
Celltrion recently announced it has received approval from the U.S. Food and Drug Administration (FDA) for the use of its Inflectra (biosimilar infliximab) product for all indications of the reference product, Janssen Biotech Inc.’s Remicade (infliximab), originally licensed in 1998. Inflectra is sold in Europe as Remsima.
Inflectra is the first biosimilar monoclonal antibody (mAb) medication and second biosimilar overall to receive FDA approval. Inflectra is indicated for the treatment of patients with a range of serious autoimmune diseases. Administered by intravenous infusion, Inflectra is approved for prescription by healthcare professionals for treating:
• Adult patients and pediatric patients (ages 6 and older) with moderately to severely active Crohn’s disease, who have had an inadequate response to conventional therapy;
• Adult patients with moderately to severely active ulcerative colitis, who have had an inadequate response to conventional therapy;
• Patients with moderately to severely active rheumatoid arthritis in combination with methotrexate;
• Patients with active ankylosing spondylitis (arthritis of the spine);
• Patients with active psoriatic arthritis;
• Adult patients with chronic severe plaque psoriasis.
 “Biosimilars can provide access to important treatment options for patients who need them,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research. “Patients and the health care community can be confident that biosimilar products are high quality and meet the agency’s rigorous scientific standards.”
“Biosimilars can provide access to important treatment options for patients who need them,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research. “Patients and the health care community can be confident that biosimilar products are high quality and meet the agency’s rigorous scientific standards.”
The FDA notes that biological products are generally derived from living organisms, and can derive from many sources, including humans, animals, microorganisms, or yeast.
The agency describes a biosimilar product as a biological product that is approved based on demonstration that it is highly similar to an already approved biological product, known as a reference product — in Inflectra’s case, Remicade.
Biosimilars, sometimes known as follow-on biologics, allow physicians to treat patients with advanced medications at more affordable prices. A true biosimilar must be shown to have no clinically meaningful differences in terms of safety, efficacy, and quality from the previously licensed reference product, with only minor differences in clinically inactive components allowed.
In a nutshell, a biosimilar product can only receive FDA approval if it has the same mechanism or mechanisms of action [to the extent that the mechanism(s) of action are known for the reference product], route(s) of administration, dosage form(s) and strength(s), and only for the indication(s) and condition(s) of use that have been approved for the reference product. Facilities in which biosimilars are manufactured must also meet FDA standards.
Biosimilar designation is determined based on the totality of evidence across extensive testing. The FDA says its approval of Inflectra was based on a review of evidence that included structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data. Inflectra was approved as a biosimilar, not as an interchangeable product.
Celltrion says development of biosimilars is especially challenging due to their molecular complexity and regulatory requirement for highly advanced manufacturing capability. The company observes that chemically based medicines are somewhat easily replicated in generic versions, but monoclonal antibodies are made up of identical antibody molecules that are extremely complex structurally and difficult to replicate, and proving bio-equivalency or bio-comparability of biosimilar products is an extensive and sophisticated task.
The company points to its employment of modern analytical tools, like High Performance Liquid Chromatography (HPLC) and Mass Spectroscopy (MS), which allow confirmation of identical protein structures, while comparability of tertiary structures — along with range of isoforms — are measured and confirmed through scientific methods. More importantly, Celltrion emphasizes that the level of comparability in biological activity is required to be proven using a range of biological assays, and clinical studies of biosimilar products are conducted to achieve appropriate data evidence for establishing comparability in pharmacokinetics, pharmacodynamics, efficacy, and safety to reference products.
Biosimilar designation is made only after examining the totality of evidence across these six categories:
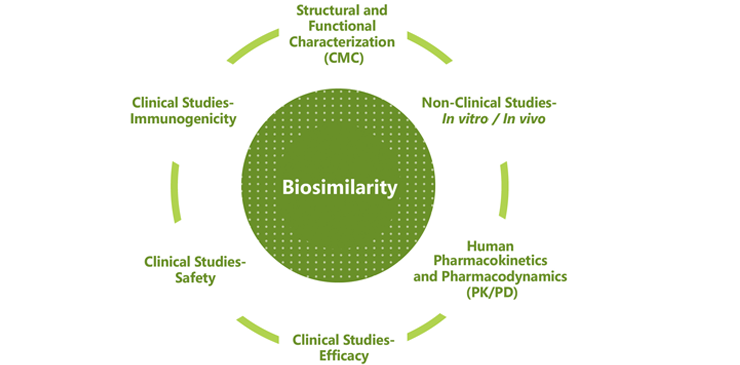
Celltrion affirms that it performs rigorous comparability tests, even before clinical trials, to confirm equivalence in vitro and ensure the quality of its medications, and that all Celltrion biosimilar monoclonal antibodies are proven equivalent in terms of quality, safety and efficacy to the respective biological reference drugs, and follow stringent biosimilar guidelines.
 “The FDA approval of Inflectra, just the second biosimilar to be approved in the U.S., is significant for the medical community as this therapy has conclusively demonstrated comparable safety and efficacy to the reference product, and will provide the medical community with an alternative, more affordable treatment option,”Vibeke Strand, MD, adjunct clinical professor, Division of Immunology/Rheumatology, Stanford University School of Medicine, said in a Celltrion press release. “This approval will help to remove barriers for the many healthcare professionals and their patients where cost and access to treatment for these chronic autoimmune diseases has been a challenge.”
“The FDA approval of Inflectra, just the second biosimilar to be approved in the U.S., is significant for the medical community as this therapy has conclusively demonstrated comparable safety and efficacy to the reference product, and will provide the medical community with an alternative, more affordable treatment option,”Vibeke Strand, MD, adjunct clinical professor, Division of Immunology/Rheumatology, Stanford University School of Medicine, said in a Celltrion press release. “This approval will help to remove barriers for the many healthcare professionals and their patients where cost and access to treatment for these chronic autoimmune diseases has been a challenge.”
The FDA’s approval of Inflectra is largely based on the totality of evidence presented at the Arthritis Advisory Committee meeting on Feb. 9, 2016, in which it was demonstrated that there are no clinically meaningful differences between Inflectra and Remicade in terms of the products safety, purity, and potency. Following discussion, the panel recommended the FDA approve Inflectra for all eligible indications by a vote of 21-3.
“As one of the first companies to navigate the biosimilar approval pathway with the FDA, we believe this approval will be an essential step in helping to clarify the application process for these critical medicines,” said HyoungKi Kim, Celltrion’s chief executive officer. “Our experience with biosimilars outside the U.S. suggests that Inflectra provides patients with both therapeutic and financial benefits, and we hope to see the same value provided in the U.S.”
Inflectra is currently approved in 71 countries, including Canada, Japan, and throughout Europe. Biosimilar infliximab was licensed by  the European Commission in September 2013 as Remsima/Inflectra for all indications of its reference product. Inflectra will be commercialized by Pfizer in the United States.
the European Commission in September 2013 as Remsima/Inflectra for all indications of its reference product. Inflectra will be commercialized by Pfizer in the United States.
Sources:
Celltrion
The U.S. Food and Drug Administration (FDA)

