In a new study entitled “Multimodal treatment of perianal fistulas in Crohn’s disease: seton versus anti-TNF versus advancement platy (PISA): study protocol for a randomized controlled trial,” authors present the protocol for a new, randomized multicenter control trial to assess the efficacy between current three lines of therapy for Crohn’s disease patients with perianal fistulas. The study was published in the journal Trials.
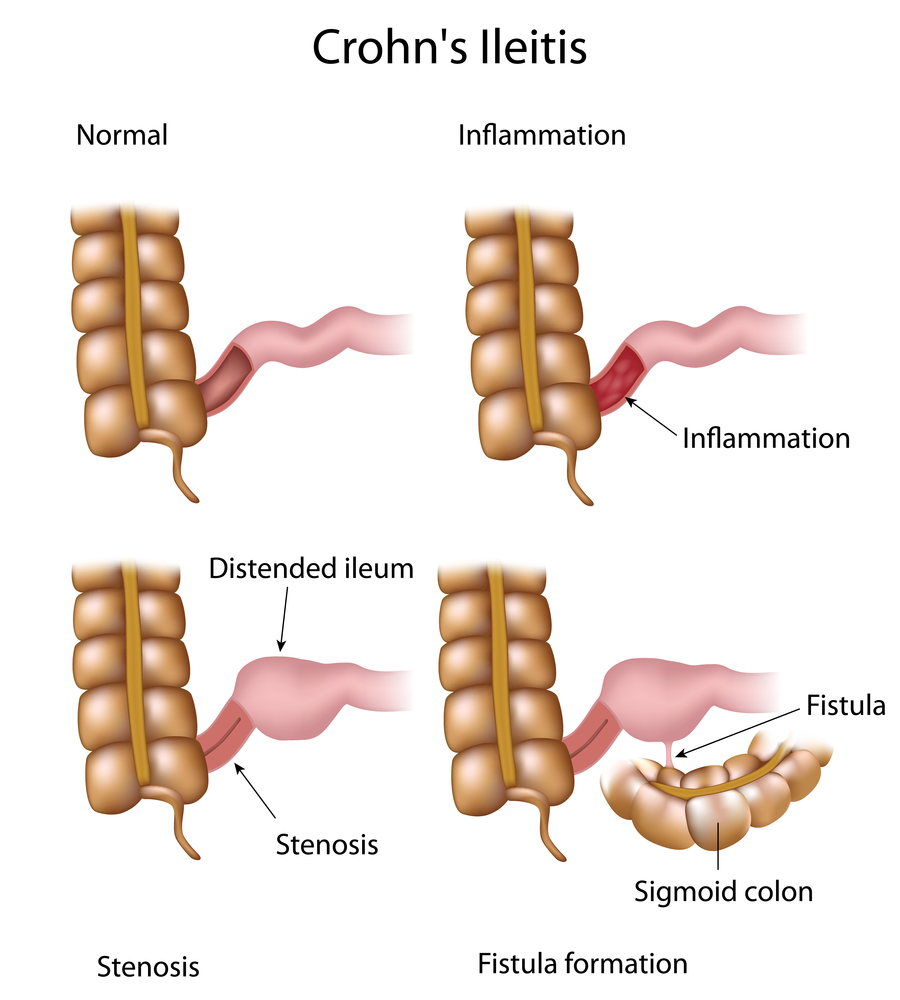
Crohn’s disease (CD), together with ulcerative colitis (UC), is the most common type of Inflammatory Bowel Disease (IBD), estimated to affect 1.4 million Americans. Up to 25% of all CD patients develop fistulas, more commonly perianal fistulas – a very disabling manifestation (associated with local pain, discharge) and source of morbidity for CD patients (including sphincter and perineal tissue destruction).
Treatment for high complex fistulas has relied in the past years on surgical procedures, either surgical seton placement for chronic drainage of the fistula or closure of the internal fistula opening by creating an advancement plasty. However, these methods were reported to negatively influence patients’ quality of life or required re-interventions, respectively. Introducing anti-TNF agents (infliximab and adalimumab) shifted patients’ treatment from surgical procedures to almost exclusive anti-TNF -based therapies. The efficacy of this treatment on the long run and its level of efficacy compared to previous surgical methods are currently unknown.
In this new study, authors will determine both efficiency and efficacy of three generally accepted therapies for high perianal fistulas in patients with CD. To this end, they designed a multicenter, randomized controlled trial (PISA trial) where patients will be randomly assigned to receive chronic seton drainage, anti-TNF treatment, or advancement plasty. As primary endpoints, the team will evaluate the number of patients who required re-intervention due to fistula-related complications (abscesses, recurrent or new tract formation) within one year. The study’s secondary endpoints included several parameters, including number of patients with closed fistulas (assessed by magnetic resonance imaging, MRI) after 18 months; disease activity (based on the Perianal Disease Activity Index, PDAI, score); number of times necessary antibiotic administration was required during fistula treatment and quality of life.
With the completion of the PISA trial, authors will determine the efficiency of the various treatment strategies, particularly on its long-term outcome effects. Up until now, patients’ enrollment was successfully achieved at 3 centers in The Netherlands, including six academic centers. Additionally, a center in Italy, Ireland and two centers in England will also participate. The trial is registered at the Nederlands Trial Register with the identifier NTR4137. Additional information and specifics on the trial can be found here.
Through the completion of the trial and result analysis, the team aims to design guidelines for treatment consensus for fistulas in CD patients.