 Cellvizio, a probe-based Confocal Laser Endomicroscopy (pCLE) system that is the flagship product of Paris, France, based Mauna Kea Technologies, should be routinely implemented to improve diagnostic evaluation and management of a number of gastric, intestinal, and biliary (liver) diseases. That’s the recommendation of a major consensus report by a team of 26 experts representing research and treatment institutions in the U.S., Europe, and Scandinavia, published in the United European Gastroenterology Journal.
Cellvizio, a probe-based Confocal Laser Endomicroscopy (pCLE) system that is the flagship product of Paris, France, based Mauna Kea Technologies, should be routinely implemented to improve diagnostic evaluation and management of a number of gastric, intestinal, and biliary (liver) diseases. That’s the recommendation of a major consensus report by a team of 26 experts representing research and treatment institutions in the U.S., Europe, and Scandinavia, published in the United European Gastroenterology Journal.
In the paper, entitled “Use of probe-based confocal laser endomicroscopy (pCLE) in gastrointestinal applications. A consensus report based on clinical evidence“ (Published online before print January 14, 2015, doi: 10.1177/2050640614566066), the coauthors note that use of the Cellvizio technology provides physicians and researchers with real-time access to histological information during standard endoscopy procedures through high-resolution cellular imaging of internal tissues.
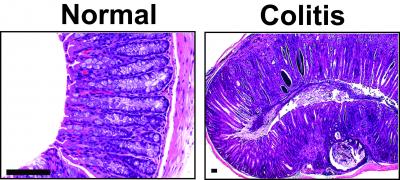
Large, international, multi-center clinical trials have demonstrated Cellvizio’s ability to help physicians detect disease more accurately in real time, to confirm absence of disease and in making immediate treatment decisions. In addition, Cellvizio can provide benefits at each step of patient management and in multiple indications, including Barrett’s Esophagus, Gastric Diseases, Bilio-pancreatic Strictures, Pancreatic Cysts, Colorectal Lesions, Lung Nodules, Urinary Tract Lesions, and Inflammatory Bowel Diseases.
The paper outlines suggested roles and uses of pCLE for evaluation and treatment of GI lesions by endoscopists, from a consensus of experts. Its aim is to provide guidance to pCLE users and to other interested parties on standardization of practice, and to suggest recommendations on training and credentialing. They say these recommendations will evolve with development of further clinical evidence and ongoing technical evolution.
Currently, the Cellvizio system incorporates miniaturized optics, optical fiber bundles, high-speed scanning and advanced imaging processing components. The system’s embedded real-time image processing software, combined with a high-speed Laser Scanning Unit (LSU), allows Cellvizio to produce sharp images at 12 frames per second. For example, Cellvizio provides a direct method to visualize the microstructure of the esophagus and differentiate normal versus abnormal Barrett’s Esophagus (BE) tissue in real-time which may improve targeted sampling, provide earlier disease detection and help patient management.
During colonoscopy procedures, standard practice is for the surgeon to remove all polyps found and send them to a pathology lab for analysis. However, pCLE with Cellvizio can magnify a polyp by a factor of 1,000, which enables the surgeon to detect cellular-level features that differentiate between cancerous and benign colorectal polyps during the colonoscopy in real time. With the additional information provided by Cellvizio, the surgeon can then exercise his or her medical judgment as to whether to leave the polyp, remove it for pathological analysis or just discard it.
The coauthors explain that this technology uses a separate unit outside the endoscope, which emits the laser illumination required for imaging (laser scanning unit or LSU), and is directed to the mucosa through a bundle of optical fibers in a small probe called a Confocal Miniprobe. The miniprobe’s tip consists of a set of lenses that enable confocal imaging with appropriate depth and resolution, and it can be introduced through the working channel of any endoscope, including the latest high-definition (HD) scopes, in order to visualize tissue at a microscopic level simultaneously with the macroscopic imaging. Depending on the organ and on the indication, several miniprobes are available, with different specifications to address the needs of each endoscopic procedure (field of view, depth of imaging and lateral resolution).
The scientists note that while eCLE provides a resolution of 0.8 microns and variable depth of focus between 0 and 250 microns, pCLE provides a resolution of up to 1 micron. Consequently they maintain that pCLE technology’s introduction as a standard element of gastroenterology procedures has resulted in significant progress being made in management strategies for a range of disorders, making an impact on many aspects of relevant areas of clinical care and requiring more standardization of practice and training. The researchers’ objective in this particular study is to provide guidance on the standardization of practice and training in Barrett’s oesophagus, biliary strictures, colorectal lesions and inflammatory bowel diseases.
For the study, initial statements were developed by five group leaders based on available clinical evidence, which were then voted on and edited by the 26 participants using a modified Delphi approach. After two rounds of votes, a statement would be validated, providing the threshold of agreement was higher than 75 Initial statements were developed by five group leaders, based on the available clinical evidence. These statements were then voted and edited by the 26 participants, using a modified Delphi approach. After two rounds of votes, statements were validated if the threshold of agreement was higher than 75 percent.
Among the 26 experts, 20 expressed their opinion on BE, 16 on biliary strictures, 16 on colorectal lesions, eight on IBD and 22 on training and credentialing. Among a total of 77 statements, 61 were adopted (79%) and 16 were rejected (21%), with those adopted justified by the grade of evidence presented.
The scientists’ consensus conclusion is that pCLE should be added as an enhancement of the diagnostic arsenal in the evaluation of the referenced indications based on its providing microscopic information that improves the diagnostic performance of the physician. They recommend that in order actually to implement this technology in the clinical routine, and to ensure good practice, standardized initial and continuing institutional training programs be established.
 “Cellvizio has taken a very important role in the evaluation of a growing number of gastrointestinal pathologies,” says the United European Gastroenterology Journal paper’s corresponding author Jean-Paul Galmiche, MD, of the division of gastroenterology and hepatology at University Hospital in Nantes, France, in a press release. “In order to standardize its use around the world, it was necessary to start developing a first set of recommendations for some key indications of the technology.”
“Cellvizio has taken a very important role in the evaluation of a growing number of gastrointestinal pathologies,” says the United European Gastroenterology Journal paper’s corresponding author Jean-Paul Galmiche, MD, of the division of gastroenterology and hepatology at University Hospital in Nantes, France, in a press release. “In order to standardize its use around the world, it was necessary to start developing a first set of recommendations for some key indications of the technology.”
The Cellvizio system has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and the European CE-Mark for use in patients’ GI and pulmonary tracts. More than 300 physicians and researchers working at more than 200 international hospitals and research centers use the Cellvizio system. Over 10,000 patients have undergone endoscopy procedures where Cellvizio was used according to Mauna Kea Technologies.
For further information on Cellvizio and/or Mauna Kea Technologies, visit:
http://www.maunakeatech.com
Sources:
Mauna Kea Technologies
United European Gastroenterology Journal
Wikipedia
Image Credits:
Mauna Kea Technologies
University Hospital, Nantes