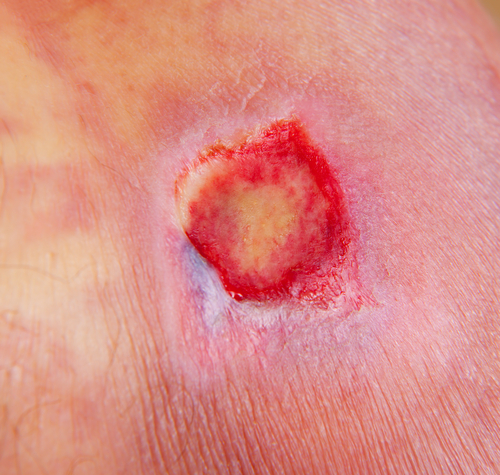
XOMA Corporation, a company dedicated to developing antibody-based therapeutics, has begun patient enrollment for a pivotal phase III study of gevokizumab for the treatment of active pyoderma gangrenous (PG), a rare neutrophilic dermatosis of expanding necrotic skin ulcers. The study is meant to evaluate the efficacy and safety of the compound in treating active ulcers that develop as a result of the rare and debilitating illness, which was granted the orphan drug designation status by the U.S. Food and Drug Administration last February.
XOMA Corporation, a company dedicated to developing antibody-based therapeutics, has begun patient enrollment for a pivotal phase III study of gevokizumab for the treatment of active pyoderma gangrenous (PG), a rare neutrophilic dermatosis of expanding necrotic skin ulcers. The study is meant to evaluate the efficacy and safety of the compound in treating active ulcers that develop as a result of the rare and debilitating illness, which was granted the orphan drug designation status by the U.S. Food and Drug Administration last February.
The company is seeking to enroll 58 patients suffering from active PG, to participate in the phase III randomized, placebo-controlled study and be administrated either with gevokizumab 60 mg once a moth or placebo, subcutaneously. The patients will continue with their treatment regimens of low-dose corticosteroids and/or immunosuppressants, as prescribed by their physicians. The researchers established as primary endpoint the complete closure of the PG target ulcer, verified at the 16th day and confirmed two weeks later, at the 140th day.
“We have reached another important milestone in our gevokizumab development activities with the launch of the first of two pivotal gevokizumab studies in pyoderma gangrenosum, one of the indications under the neutrophilic dermatoses umbrella for which there are no available therapies approved by the FDA,” said the senior vice-president of research and development and chief medical officer at XOMA, Paul Rubin, MD.
Being a monoclonal antibody with specific allosteric modulating properties, Gevokizumab aims to treat several inflammatory diseases. It blocks the pro-inflammatory cytokine interleukin-1 beta (IL-1 beta), as well as modulates the cellular signaling events responsible for the production of inflammation.
“Patients with PG experience deep and painful skin ulcers that often become chronic wounds. In our pilot study, five of the six patients enrolled responded to gevokizumab, and four experienced complete wound closure by three months. We believe gevokizumab has the potential to help PG patients reduce the amount of time it takes to heal from this painful and unsightly condition. With the first U.S. only study open for enrollment, we will complete the necessary steps to open the second pivotal PG Phase 3 study, which will include both U.S. sites and centers outside of the United States,” Rubin added.
XOMA expects to reduce the burden of patients suffering from pyoderma gangrenosum, which affects approximately 1 in every 100,000 people, according to the U.S. Department of Health and Human Services’ National Institutes of Health’s Office of Rare Disease Research. Diagnosed patients number between 11,000 and 14,000 in the United States alone, and the most common systemic underlying causes for it are ulcerative colitis and Crohn’s disease.