 SQI Diagnostics Inc., a company that designs and commercializes technology for advanced microarray diagnostics, recently announced that the FDA has given approval for the commercialization of its product, a Celiac Panel, in the United States. This new technology will allow for improved celiac disease-affected individuals’ diagnosis.
SQI Diagnostics Inc., a company that designs and commercializes technology for advanced microarray diagnostics, recently announced that the FDA has given approval for the commercialization of its product, a Celiac Panel, in the United States. This new technology will allow for improved celiac disease-affected individuals’ diagnosis.
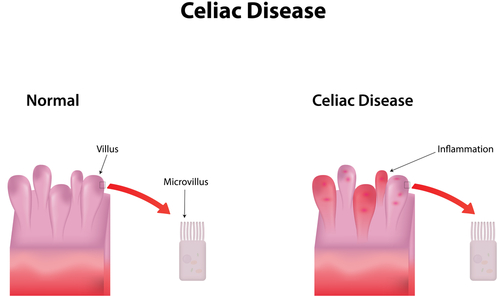
Celiac disease is an autoimmune disease of the small intestine where the body reacts against gliadin, a gluten protein, and is characterized by an impaired absorption capacity of key nutrients. According to the American Journal of Gastroenterology, up to 97% of celiac-affected individuals remain undiagnosed, making it a much more common disease than current statistics reveal.
The SQI Celiac Panel is an in vitro diagnostic test to determine, in human serum, the amount of IgA and IgG immunoglobulins of antibodies generated by the body against deamidated gliadin peptide (DGP) and tissue transglutaminase (tTG). This system was designed to be used in a sqid-XTM system and will help in the diagnosis of celiac disease, together with the current test for anti-tTG and anti-DPG. The development of the panel is a promising step in the right direction for those with celiac disease, as diagnostic and therapeutic products have lagged in the biotech and healthcare spheres.
Andrew Morris, CEO, SQI Diagnostics noted, “We are very excited to have received FDA clearance. This clearance lays the foundation for sales in the US market of our newest in vitro diagnostic (IVD) autoimmune test. Our revenue growth during the last fiscal year was achieved through the development of custom multiplexed assays for pharmaceutical companies and their clinical research organizations and most recently, molecular testing in the animal and human health markets. This clearance demonstrates the Company’s ability to achieve success in the third multiplexing market in which it is involved: IVD autoimmune test development. The clearance is also an important element in marketing our products and services with leading global pharmaceutical companies, and diagnostic partners in our newest area of molecular testing.”
The company is currently developing other in vitro diagnosis devices for lupus, vasculitis and vasculitis, a quantitative 12-plex, 3-plex and 8-plex panel, respectively.