 Animal study results reported by researchers at the University at Buffalo School of Medicine and Biomedical Sciences and covered in a UB News release show that a new oral biologic medication has successfully treated chronic, precancerous intestinal inflammation.
Animal study results reported by researchers at the University at Buffalo School of Medicine and Biomedical Sciences and covered in a UB News release show that a new oral biologic medication has successfully treated chronic, precancerous intestinal inflammation.
Featured on the cover of the current issue of the American Association for Cancer Research’s eponymously-named journal Cancer Research, and published online ahead of print in September, the UB study, whose first author is a UB MD/PhD student, the study’s findings are characterized by the journal’s editors as “striking.”
Entitled “Oral Interleukin-10 Alleviates Polyposis via Neutralization of Pathogenic T-Regulatory Cells” (Cancer Res October 1, 2014 74:5377-5385; Published Online First September 16, 2014; dpi:10.1158/0008-5472.CAN-14-0918 ), the study is coauthored by Allen Y. Chung, Qingsheng Yijun, Sun Li, Thomas F. Conway, Lauren P. Virtuoso, Nicholas G. Battaglia, Sarah J. Blair, and Nejat K. Egilmez of the Department of Microbiology and Immunology at the University at Buffalo School of Medicine and Biomedical Sciences, Buffalo, New York; Magdia De Jesus and Nicholas J. Mantis of the Division of Infectious Diseases, Wadsworth Center, Albany, New York; Kristen L. Dennis and Khashayarsha Khazaie of the Division of Gastroenterology, Northwestern University Feinberg School of Medicine, Chicago, Illinois; Charles LeVea of the Department of Surgical Pathology, Roswell Park Cancer Institute, Buffalo, New York; Jin Yao of the Department of Electrical and Computer Engineering, University of Florida, Gainesville, Florida (also associated with UB); and Stacia Furtado and Edith Mathiowitz of the Department of Molecular Pharmacology, Physiology & Biotechnology, Brown University, Providence, Rhode Island. K. Khazaie is now with Department of Immunology, Mayo Clinic College of Medicine, Rochester, Minnesota, and Q. Li and N.K. Egilmez with the Department of Microbiology and Immunology, University of Louisville School of Medicine, Louisville, Kentucky.
The coauthors observe that immune dysregulation drives the pathogenesis of chronic inflammatory, autoimmune, and dysplastic disorders, and that while often intended to address localized pathology, most immune modulatory therapies are administered systemically and carry inherent risk of multiorgan toxicities.
In this study they demonstrate, in a murine model of spontaneous gastrointestinal polyposis, that site-specific uptake of orally administered IL10 microparticles ameliorates local and systemic disease to enhance survival.
They report that mechanistic investigations revealed that the therapeutic benefit of this treatment derives from neutralization of disease-promoting FoxP3+RoRt+IL17+ pathogenic T-regulatory cells (pgTreg), with a concomitant restoration of FoxP3+RoRtIL17 conventional T-regulatory cells (Treg). These findings provide a proof-of-principle for the ability of an oral biologic to restore immune homeostasis at the intestinal surface. Furthermore, they implicate local manipulation of IL10 as a tractable therapeutic strategy to address the inflammatory sequelae associated with mucosal premalignancy.
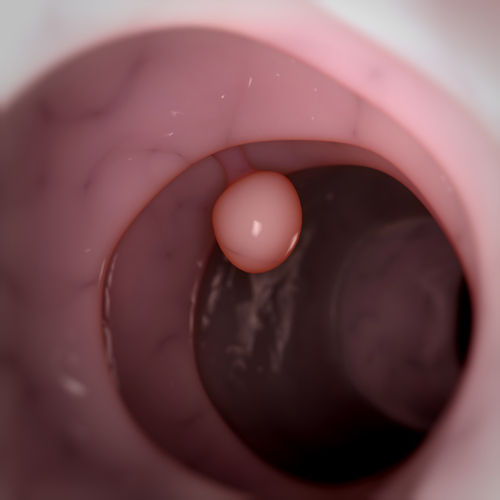
A UB release notes that Inflammatory cells in the colon, called polyps, are very common after age 50, and that the average 60-year-old has an estimated 25 percent chance of having polyps. Most polyps are benign, but some will develop into colon cancer, and for that reason, standard treatment for polyps, usually detected by colonoscopy, is to remove them.
 “Our most important finding is that disease-promoting inflammatory cells in the mouse intestine can be targeted by oral formulations of purified cellular proteins, rendering the inflammatory cells less able to cause disease, says study first author Allen Chung, an MD/PhD student in the UB medical school’s Medical Scientist Training Program.
“Our most important finding is that disease-promoting inflammatory cells in the mouse intestine can be targeted by oral formulations of purified cellular proteins, rendering the inflammatory cells less able to cause disease, says study first author Allen Chung, an MD/PhD student in the UB medical school’s Medical Scientist Training Program.
According to the paper, this is one of the first instances of an oral biologic being used successfully to alter the natural history of a genetic disease; specifically in this case, a genetic mutation that puts affected individuals at very high risk for developing colon cancer.
The UB release explains that oral biologics are drugs based on biological molecules derived from natural substances sourced in humans, animals or microorganisms. These agents have obvious advantages over other biologics, which must be injected or otherwise administered.
Site-specific oral therapy for intestinal disorders is a promising avenue of research, says Mr. Chung, since it avoids the use of systemic circulation and therefore minimizes the toxic side effects inherently associated with such a mode of drug delivery most notably, liver, kidney and brain toxicity.
However, even though the drug used in the study can be administered orally without threat of potential toxicities associated with exposure to kidney, liver and brain tissue, it did not deliver its pharmaceutical payload until it reached the intestinal surface.
“We found that high pharmacologic levels of the bioactive drug of interest can be achieved at the intestinal surface without systemic circulation of the drug,” Mr. Chung explains in the release, “so the drug can be administered without the threat of potential toxicities associated with exposure to kidney, liver and brain tissue.”
Using animal models of precancerous polyps in the bowel, Mr. Chung and his team determined that certain types of immune cells within a chronically inflamed intestine can become “rewired,” causing them to paradoxically contribute to disease development rather than protect against it.
The researchers proceeded to reprogram these immune cells, making them lose their pathogenic potential, which they achieved by delivering immuno-modulatory compounds — specifically, the bioactive protein interleukin-10 — into the inflamed intestine, thereby reducing both speed and severity of polyp formation. Interleukin- 10 was administered as a whole recombinant protein encapsulated within polymer micro-particles, a process originally developed by some of Mr. Chung’s collaborators at Brown University — Investigator in Molecular Pharmacology, Physiology & Biotechnology Stacia Furtado, and Professor of Medical Science and Engineering in the Brown Department of Molecular Pharmacology, Physiology & Biotechnology Edith Mathiowitz.
10 was administered as a whole recombinant protein encapsulated within polymer micro-particles, a process originally developed by some of Mr. Chung’s collaborators at Brown University — Investigator in Molecular Pharmacology, Physiology & Biotechnology Stacia Furtado, and Professor of Medical Science and Engineering in the Brown Department of Molecular Pharmacology, Physiology & Biotechnology Edith Mathiowitz.
This, in turn, significantly benefited the mice, relieving symptoms including anemia, enlarged spleen and weight loss, and lengthening their lifespan.
Site-specific oral therapy for intestinal disorders is a promising avenue of research, says Mr. Chung, whose findings in this study formed the basis for his UB doctoral thesis.
Allen Chung became interested in exploring the relationship between inflammation and polyp formation as a PhD candidate in UB’s Department of Microbiology and Immunology. He began investigating how immunologic activity in the intestine may contribute to the development of precancerous polyps or polyposis within the bowel.
“It has long been known that inflammation within the colon increases the risk of developing colon cancer,” says Mr. Chung. “Rates of colon cancer are much higher in individuals with inflammatory bowel diseases, such as Crohns disease or ulcerative colitis, than in the overall population.”
“There are also genetic mutations that predispose individuals to develop colon cancer in early adulthood and these individuals have been found to develop intestinal inflammation even before the appearance of their neoplastic disease,” he says.
 Mr. Chung and his co-author and doctoral mentor Nejat Egilmez, PhD, formerly of UB and now at the University of Louisville, have applied for patent protection on the research.
Mr. Chung and his co-author and doctoral mentor Nejat Egilmez, PhD, formerly of UB and now at the University of Louisville, have applied for patent protection on the research.
“We are now involved in studies which hope to determine whether some of the immunologic phenomena we observed in our mouse models are representative of intestinal disease in human patients who harbor genetic mutations which predispose them to develop colon cancer,” says Mr. Chung, who is currently completing his medical education at UB and expects to graduate with his combined MD/PhD in 2016. He plans to pursue residency training in internal medicine, pediatrics or obstetrics-gynecology.
The work was funded by the National Institutes of Health and the Howard Hughes Medical Institute.
Sources:
University of Buffalo
Cancer Research
Image Credits:
University of Buffalo